ARS Pharmaceuticals Submits sNDA to FDA for neffy® 1 mg Dose for Pediatric Patients with Type I Allergic Reactions Who Weigh 15 to 30 kg (33-66 lbs.)
09 Setembro 2024 - 5:47PM

ARS Pharmaceuticals, Inc. (Nasdaq: SPRY), a
biopharmaceutical company dedicated to empowering at-risk patients
and their caregivers to better protect patients from severe
allergic reactions that could lead to anaphylaxis, announced today
the submission of a supplemental New Drug Application (sNDA) for
neffy® 1 mg for
the treatment of Type I Allergic Reactions, including anaphylaxis,
in children who weigh 15 to 30 kg (33-66 lbs.). Pharmacokinetic
(PK) data for
neffy 1mg were
slightly higher than that of adults who receive the same dose and
pharmacodynamic (PD) response was similar.
Food allergies affect approximately six million children in the
U.S., of which more than 40 percent have experienced a severe
allergic reaction such as anaphylaxis. Additionally, studies show
that two out of three children are afraid of needles and more than
half of parents express fear about using their child’s epinephrine
auto-injector – a potentially lifesaving treatment.
“Fear of needles is a common reason children refuse treatment
with auto-injectors. If approved, neffy
1mg will be the first needle-free epinephrine
option for younger children, in addition to being the first new
delivery method for this population in more than 35 years,” says
Richard Lowenthal, Co-Founder, President and CEO of ARS
Pharmaceuticals. “Nearly half of all current epinephrine
auto-injector prescriptions are for children, and more than half of
those are for children weighing 15 to 30 kg (33-66 lbs.). We
believe there is a clear need for innovative treatments like
neffy 1mg.”
Highlights from the data submitted to the FDA underscore several
key attributes:
- No risk of needle-related adverse
events including accidental injection into the hand or fingers of a
child or caregiver. This occurs approximately 3,500 times each year
in the U.S. with epinephrine injection devices and
disproportionately affects 15 to 30 kg (33-66 lb.) pediatric
patients.
- Simple insert and press mechanism
delivers epinephrine almost instantly without any hold time
required in the nose.
- Human factor studies showed that
adults without knowledge of the disease or device (e.g. a
babysitter or a teacher), were able to successfully use the device
by simply reading the instructions. These studies also showed that
untrained children as young as 10 years of age can easily use
neffy with self-administration.
- neffy allows for
temperature exposure up to 122°F (50°C). If accidentally frozen, it
can be thawed and administered.
“There is no doubt that this innovation is going to save lives.
Patients need options, and neffy is helping to
solve that issue,” said Eleanor Garrow-Holding, President and CEO
of the Food Allergy & Anaphylaxis Connection Team (FAACT), a
national patient advocacy group raising awareness for all
individuals affected by food allergies and life-threatening
anaphylaxis. “There has been little advancement in emergency
treatments for this community, especially to address concerns about
children carrying and using an epinephrine auto-injector. For the
safety of our children with food allergies, making
neffy 1mg available as soon as
possible must be a high priority.”
About Type I Allergic Reactions including
AnaphylaxisType I severe allergic reactions are serious
and potentially life-threatening events that can occur within
minutes of exposure to an allergen and require immediate treatment
with epinephrine, the only FDA-approved medication for these
reactions. While epinephrine auto-injectors have been shown to be
highly effective, there are well published limitations that result
in many patients and caregivers delaying or not administering
treatment in an emergency situation. These limitations include fear
of the needle, lack of portability, needle-related safety concerns,
lack of reliability, and complexity of the devices. There are
approximately 40 million people in the United States who experience
Type I severe allergic reactions due to food, venom or insect
stings. Of those, only 3.3 million currently have an active
epinephrine auto-injector prescription, and of those, only half
consistently carry their prescribed auto-injector. Even if patients
or caregivers carry an auto-injector, more than half either delay
or do not administer the device when needed in an emergency.
About ARS Pharmaceuticals, Inc.ARS Pharma
is a biopharmaceutical company dedicated to empowering at-risk
patients and their caregivers to better protect patients from
severe allergic reactions that could lead to anaphylaxis. The
Company is
commercializing neffy® 2mg
(trade name EURneffy in the EU)
(previously referred to as ARS-1), an epinephrine nasal spray for
patients 30 kg or greater with Type I allergic reactions including
food, medications and insect bites that could lead to
life-threatening anaphylaxis. For more information,
visit www.ars-pharma.com.
Forward-Looking StatementsStatements in this
press release that are not purely historical in nature are
“forward-looking statements” within the meaning of the Private
Securities Litigation Reform Act of 1995. These statements include
but are not limited to: the potential market and demand for
neffy; the needle-free profile of
neffy potentially increasing the likelihood that
patients and caregivers may carry and administer epinephrine;
neffy’s potential benefits to patients and
caregivers; and other statements that are not historical fact.
Because such statements are subject to risks and uncertainties,
actual results may differ materially from those expressed or
implied by such forward-looking statements. Words such as “can,”
“could,” “potential,” “will,” and similar expressions are intended
to identify forward-looking statements. These forward-looking
statements are based upon ARS Pharma’s current expectations and
involve assumptions that may never materialize or may prove to be
incorrect. Actual results and the timing of events could differ
materially from those anticipated in such forward-looking
statements as a result of various risks and uncertainties, which
include, without limitation: the ability to obtain and maintain
regulatory approval for neffy; potential
safety and other complications from neffy;
the labelling for neffy in any future
indication or patient population, if approved; the scope, progress
and expansion of developing and
commercializing neffy; the potential for
payors to delay, limit or deny coverage for neffy;
the size and growth of the market therefor and the rate and degree
of market acceptance thereof vis-à-vis intramuscular injectable
products; ARS Pharma’s ability to protect its intellectual property
position; and the impact of government laws and regulations.
Additional risks and uncertainties that could cause actual outcomes
and results to differ materially from those contemplated by the
forward-looking statements are included under the caption “Risk
Factors” in ARS Pharma’s Quarterly Report on Form 10-Q for the
quarter ended June 30, 2024, filed with the Securities
and Exchange Commission (SEC) on August 6, 2024. This and
other documents ARS Pharma files with the SEC can also be
accessed on ARS Pharma’s website at ir.ars-pharma.com by
clicking on the link “Financials & Filings” under the
“Investors & Media” tab.
ARS Investor Contact:Justin ChakmaARS
Pharmaceuticalsjustinc@ars-pharma.com
ARS Media Contact:Christy CurranSam Brown
Inc.615.414.8668christycurran@sambrown.com
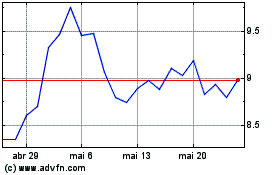
ARS Pharmaceuticals (NASDAQ:SPRY)
Gráfico Histórico do Ativo
De Out 2024 até Nov 2024
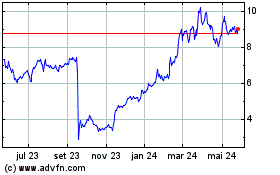
ARS Pharmaceuticals (NASDAQ:SPRY)
Gráfico Histórico do Ativo
De Nov 2023 até Nov 2024