AIM ImmunoTech Granted U.S. Patent for Ampligen® for the Treatment of Endometriosis
03 Outubro 2024 - 9:45AM

AIM ImmunoTech Inc. (NYSE American:
AIM) (“AIM” or the “Company”) today announced that
the United States Patent and Trademark Office (USPTO) has granted
U.S. patent No. 12,102,649, covering both compositions and methods
comprising the Company’s drug Ampligen® in the treatment of
endometriosis, a painful chronic condition that affects nearly 10%
of women of reproductive age, or approximately 6.5 million women in
the United States.
Endometriosis is a disease in which tissue
similar to the lining of the uterus grows outside the uterus,
causing severe pelvic pain and making it difficult or impossible to
become pregnant. It can cause inflammation and infertility, and
symptoms such as intense menstrual cramps, severe pelvic pain and
chronic fatigue, all of which can significantly impact quality of
life. Despite its prevalence, treatment options are limited, and
many women experience years of misdiagnosis.
The patented method involves the administration
of a therapeutically effective amount of a pharmaceutical
composition containing AIM’s proprietary double-stranded RNA
products.
The patent’s claims describe a unique class of
therapeutic double-stranded RNAs (e.g., tdsRNA, Ampligen®, rugged
dsRNA) which are key components in these potentially groundbreaking
treatment methods and compositions. The versatile administration
options offer flexibility for patient-specific needs and care. The
patent also covers treatments targeting recurrent endometriosis and
includes options for co-administration with interferons, including
well-known types such as alpha and beta interferons.
Patients with endometriosis also are at more
than four times the risk of developing ovarian cancer than are
people without endometriosis, according to a recent article in
JAMA. As we have previously announced, Ampligen has also shown
positive results as part of a combination therapy in the treatment
of advanced recurrent ovarian cancer.
Read more: “AIM ImmunoTech Announces Positive
Top-Line, Protocol-Planned Interim Report Data from the Study of
Ampligen Combined with Pembrolizumab for the Treatment of Recurrent
Ovarian Cancer”
Robert Edwards, MD, a world-renowned expert in
women’s reproductive health and Chair of Ob/Gyn/RS at the
University of Pittsburgh Medical Center, Magee-Womens Hospital,
stated: “Endometriosis is a chronic disease that threatens the
fertility and wellbeing of greater than 10% of the population, but
total incidence is not known. Effective treatment now involves some
form of estrogen deprivation or surgery, both of which have serious
side effects and fertility risks themselves. A medical therapy that
relieves symptoms and abates the inflammatory response would be a
significant breakthrough in treatment options. At UPMC we have
conducted an extensive clinical evaluation of Ampligen in recurrent
ovarian cancer with positive results. Ampligen’s safety profile is
well developed and Ampligen has been generally well tolerated.”
AIM Chief Executive Officer Thomas K. Equels
commented, "This patent is a significant milestone for AIM, not
only strengthening our intellectual property portfolio, but also
expanding our potential to provide life-changing therapies for
women suffering from endometriosis. With the global focus on
women's health, we are excited about the possibilities this new
treatment presents. At AIM, our goal is to make a difference by
improving the quality of life for women suffering from
endometriosis."
About AIM ImmunoTech Inc.
AIM ImmunoTech Inc. is an immuno-pharma company
focused on the research and development of therapeutics to treat
multiple types of cancers, immune disorders and viral diseases,
including COVID-19. The Company’s lead product is a first-in-class
investigational drug called Ampligen® (rintatolimod), a dsRNA
and highly selective TLR3 agonist immuno-modulator with broad
spectrum activity in clinical trials for globally important
cancers, viral diseases and disorders of the immune system.
For more information, please
visit aimimmuno.com and connect with the Company
on X, LinkedIn, and Facebook.
Cautionary Statement
This press release contains forward-looking
statements within the meaning of the Private Securities Litigation
Reform Act of 1995 (the “PSLRA”). Words such as “may,” “will,”
“expect,” “plan,” “anticipate,” “continue,” “believe,” “potential,”
“upcoming” and other variations thereon and similar expressions (as
well as other words or expressions referencing future events or
circumstances) are intended to identify forward-looking statements.
Many of these forward-looking statements involve a number of risks
and uncertainties. Data, pre-clinical success and clinical success
seen to date does not guarantee that Ampligen will be approved as a
therapy for endometriosis or ovarian cancer. The Company urges
investors to consider specifically the various risk factors
identified in its most recent Form 10-K, and any risk factors or
cautionary statements included in any subsequent Form 10-Q or Form
8-K, filed with the U.S. Securities and Exchange Commission. You
are cautioned not to place undue reliance on these forward-looking
statements, which speak only as of the date of this press release.
Among other things, for those statements, the Company claims the
protection of the safe harbor for forward-looking statements
contained in the PSLRA. The Company does not undertake to update
any of these forward-looking statements to reflect events or
circumstances that occur after the date hereof.
Photos accompanying this announcement are available
at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/b3aac790-f746-4dac-afe7-796d4ce31897
https://www.globenewswire.com/NewsRoom/AttachmentNg/c697332b-3cca-4968-8f85-b1a98a8882bd
Investor Contact:
JTC Team, LLC
Jenene Thomas
(908) 824-0775
AIM@jtcir.com
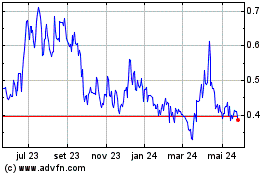
AIM ImmunoTech (AMEX:AIM)
Gráfico Histórico do Ativo
De Out 2024 até Nov 2024
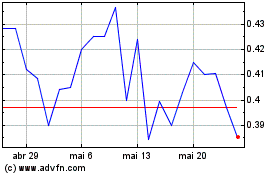
AIM ImmunoTech (AMEX:AIM)
Gráfico Histórico do Ativo
De Nov 2023 até Nov 2024