- First-of-its-kind randomized controlled trial comparing
computer assisted vacuum thrombectomy (CAVT) using Penumbra's
Lightning Flash™ with anticoagulation versus anticoagulation
alone
- Penumbra's Indigo® Aspiration with Lightning
portfolio offers the only CAVT technologies currently available in
the U.S. and Lightning Flash is the latest advancement designed to
allow physicians to remove large blood clots in the body safely,
simply and with speed
ALAMEDA,
Calif., Nov. 28, 2023 /PRNewswire/ -- Penumbra,
Inc. (NYSE: PEN), a global healthcare company focused on innovative
therapies, announced the first patient enrolled in STORM-PE, a
first-of-its-kind prospective, multi-center, randomized controlled
trial evaluating anticoagulation alone vs anticoagulation plus
Lightning Flash™ for the treatment of pulmonary embolism (PE).
In partnership with PERT Consortium™, a multi-disciplinary
group dedicated to improving the care of patients with PE, the
STORM-PE trial aims to advance the understanding of the role of
computer assisted vacuum thrombectomy (CAVT) in the management of
acute PE, with a goal of improving the outcomes for patients with
this life-threatening condition.
"STORM-PE is the first head-to-head trial comparing
anticoagulation, the mainstay and standard of care for treating
acute PE, to CAVT," said Rachel
Rosovsky, MD, MPH, co-global principal investigator of
STORM-PE, hematologist and clinical investigator at the
Massachusetts General Hospital. "We have already seen the
significant impact Lightning Flash can have on patients. The
results generated from this pivotal RCT study will provide level 1
evidence of how CAVT compares to current treatment paradigms and
will inform us if treatment guidelines need to include this
technology as a frontline therapeutic option in patients with
intermediate high-risk PE."
STORM-PE will enroll up to 100 participants at up to 20 sites.
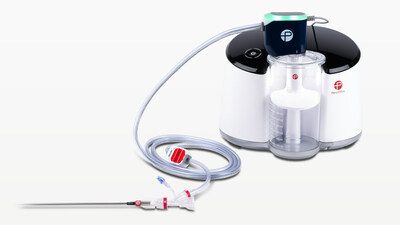
Penumbra's Lightning Flash can be used to remove emboli and thrombi
from vessels of the peripheral arterial and venous systems and can
be used for the treatment of PE. Lightning Flash combines
hypotube-based Indigo Aspiration Catheters with
LightningTM Intelligent Aspiration, a unique computer
assisted clot detection technology that can differentiate between
clot and blood, designed to reduce blood loss and the need for
clot-dissolving drugs, which may lower the risk of bleeding
complications.
"Enrolling the first patient in STORM-PE RCT is an important
milestone," said Nicolas J. Mouawad,
MD, MPH, MBA, RPVI, chief of vascular and endovascular surgery,
McLaren Health Care, Bay City, MI.
"This landmark RCT will be instrumental to advancing the standard
of care for patients with pulmonary embolism."
"Endovascular therapy has been shown to be an increasingly safe
and effective treatment for patients with a symptomatic acute
pulmonary embolism," said Robert
Lookstein, MD, MHCDL co-global principal investigator and
professor of radiology and surgery at the Icahn School of Medicine
at Mount Sinai. "We are proud to commence enrollment in the
STORM-PE trial. This trial is designed to answer whether CAVT
plus anticoagulation can restore normal function to the failing
right ventricle faster and more reliably than anticoagulation
alone. We are confident this trial will generate foundational data
leading to the next generation of randomized studies in the field
of venous thromboembolism for years to come."
In the U.S., an estimated 900,000 cases of symptomatic PE occur
annually[i]. Pulmonary embolism can be life-threatening with 10-30
percent of individuals dying within one month of
diagnosisi.
"Our commitment to clinical research and innovation enables us
to lead with insight and continue to pioneer interventional
therapies that have a significant impact on patients such as our
CAVT technologies," said James F.
Benenati, M.D., FSIR, chief medical officer at Penumbra. "In
partnership with PERT Consortium, we are committed to generating
robust clinical evidence that will help transform care so patients,
especially those with serious conditions such as PE, can return
home quickly and live fully."
About Penumbra
Penumbra, Inc., headquartered in
Alameda, California, is a global
healthcare company focused on innovative therapies. Penumbra
designs, develops, manufactures and markets novel products and has
a broad portfolio that addresses challenging medical conditions in
markets with significant unmet need. Penumbra supports healthcare
providers, hospitals and clinics in more than 100 countries. For
more information, visit www.penumbrainc.com and connect
on Twitter and LinkedIn.
Important Safety Information
Additional
information about Penumbra's products can be located on Penumbra's
website at https://www.penumbrainc.com/providers.
Prior to use, please refer to Instructions for Use for complete
product indications, contraindications, warnings, precautions,
potential adverse events and detailed instructions for use. Risk
information can be found at
http://www.peninc.info/risk.
Forward-Looking Statements
Except for historical
information, certain statements in this press release are
forward-looking in nature and are subject to risks, uncertainties
and assumptions about us. Our business and operations are subject
to a variety of risks and uncertainties and, consequently, actual
results may differ materially from those projected by any
forward-looking statements. Factors that could cause actual results
to differ from those projected include, but are not limited to:
failure to sustain or grow profitability or generate positive cash
flows; failure to effectively introduce and market new products;
delays in product introductions; significant competition; inability
to further penetrate our current customer base, expand our user
base and increase the frequency of use of our products by our
customers; inability to achieve or maintain satisfactory pricing
and margins; manufacturing difficulties; permanent write-downs or
write-offs of our inventory; product defects or failures;
unfavorable outcomes in clinical trials; inability to maintain our
culture as we grow; fluctuations in foreign currency exchange
rates; potential adverse regulatory actions; and the potential
impact of any acquisitions, mergers, dispositions, joint ventures
or investments we may make. These risks and uncertainties, as well
as others, are discussed in greater detail in our filings with the
Securities and Exchange Commission (SEC), including our Annual
Report on Form 10-K for the year ended December 31, 2022 filed with the SEC on
February 23, 2023. There may be
additional risks of which we are not presently aware or that we
currently believe are immaterial which could have an adverse impact
on our business. Any forward-looking statements are based on our
current expectations, estimates and assumptions regarding future
events and are applicable only as of the dates of such statements.
We make no commitment to revise or update any forward-looking
statements in order to reflect events or circumstances that may
change.
|
Contact
|
|
|
|
|
Jennifer
Heth
|
Parinaz
Farzin
|
|
Penumbra,
Inc.
|
Merryman
Communications
|
|
jheth@penumbrainc.com
|
parinaz@merrymancommunications.com
|
|
510-995-9791
|
310.600.6746
|
i "Learn about Pulmonary Embolism,"American Lung
Association. Accessed on Oct. 26,
2023.
https://www.lung.org/lung-health-diseases/lung-disease-lookup/pulmonary-embolism/learn-about-pulmonary-embolism
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/first-patient-in-storm-pe-rct-enrolled-evaluating-penumbras-latest-computer-assisted-vacuum-thrombectomy---lightning-flash---for-treatment-of-acute-pulmonary-embolism-301998812.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/first-patient-in-storm-pe-rct-enrolled-evaluating-penumbras-latest-computer-assisted-vacuum-thrombectomy---lightning-flash---for-treatment-of-acute-pulmonary-embolism-301998812.html
SOURCE Penumbra, Inc.