MedinCell’s partner Teva Announces Recruitment Completion of Phase 3 Clinical Study of mdc-TJK / Olanzapine Long-Acting Injectable (LAI)
09 Janeiro 2024 - 3:26PM
Business Wire
Teva Pharmaceutical Industries Ltd. announced the successful
completion of the enrollment in the EU and US of the anticipated
640 participants of the ongoing Phase 3 clinical trial of mdc-TJK
(TEV-44749) at the J.P. Morgan Healthcare Conference. Results of
the study are expected in the second half of 2024.1
mdc-TJK is an investigational once-monthly subcutaneous
long-acting injection of the atypical antipsychotic olanzapine for
the treatment of schizophrenia. It has the potential to be the
first long-acting olanzapine with a favorable safety
profile.2
Teva remains fully responsible to lead the development and
commercialization of olanzapine LAI globally.
MedinCell may receive up to $117 million in development and
commercial milestones over the coming years for mdc-TJK, and is
eligible for royalties on all net sales.
1 Full webcast of Teva’s presentation at 42nd Annual J.P. Morgan
Healthcare Conference, January 8, 2024 is available on
ir.tevapharm.com. 2 The only existing LAI of Olanzapine has a FDA
black box warning from for PDSS (Post injection Delirium/Sedation
Syndrome) that limits its use.
About MedinCell
MedinCell is a clinical- and commercial-stage biopharmaceutical
company developing long-acting injectable drugs in many therapeutic
areas. Our innovative treatments aim to guarantee compliance with
medical prescriptions, to improve the effectiveness and
accessibility of medicines, and to reduce their environmental
footprint. They combine already known and used active ingredients
with our proprietary BEPO® technology which controls the delivery
of a drug at a therapeutic level for several days, weeks or months
from the subcutaneous or local injection of a simple deposit of a
few millimeters, entirely bioresorbable. The first treatment based
on BEPO technology, intended for the treatment of schizophrenia,
was approved by the FDA in April 2023, and is now distributed in
the United States by Teva under the name UZEDY™ (BEPO technology is
licensed to Teva under the name SteadyTeq™).
We collaborate with leading pharmaceutical companies and
foundations to improve global health through new treatment options.
Based in Montpellier, MedinCell currently employs more than 140
people representing more than 25 different nationalities.
UZEDY™ and SteadyTeq™ are trademarks of Teva Pharmaceuticals
www.medincell.com
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240109013864/en/
David Heuzé - Head of Communications
david.heuze@medincell.com / +33 (0)6 83 25 21 86
Media Relations Nicolas Mérigeau
medincell@newcap.eu / +33 (0)1 44 71 94 94
Investor Relations France Louis-Victor
Delouvrier/Alban Dufumier medincell@newcap.eu / +33 (0)1 44 71
94 94
Head of US Financial Strategy & IR Grace Kim
grace.kim@medincell.com / +1 (646) 991-4023
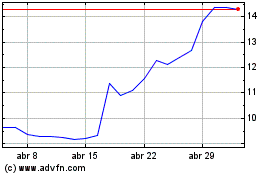
Medincell (EU:MEDCL)
Gráfico Histórico do Ativo
De Jun 2024 até Jul 2024
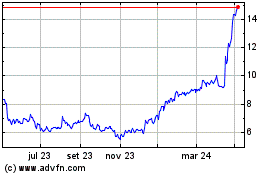
Medincell (EU:MEDCL)
Gráfico Histórico do Ativo
De Jul 2023 até Jul 2024