Alzamend Neuro Partners with Massachusetts General Hospital for a Phase II Clinical Trial of AL001, a Next‑Generation Lithium Therapeutic Drug Candidate, involving Patients with Major Depressive Disorder
12 Agosto 2024 - 9:00AM
Business Wire
- Harvard University Associate Professor, Dr. Ovidiu Andronesi
MD, PhD, will be the principal investigator of the study
- Head-to-head study of AL001 versus a marketed lithium
carbonate product will be conducted; the goal is to compare lithium
in human brain and brain structures to identify a potentially ideal
dose of AL001 that is safe and effective as current
products
Alzamend Neuro, Inc. (Nasdaq: ALZN) (“Alzamend”), a
clinical-stage biopharmaceutical company focused on developing
novel products for the treatment of Alzheimer’s disease
(“Alzheimer’s”), bipolar disorder (“BD”), major
depressive disorder (“MDD”) and post-traumatic stress
disorder (“PTSD”), today announced that it is partnering
with Massachusetts General Hospital as its contract research
organization (“CRO”) to conduct first of its kind Phase II
clinical study of AL001 for treatment of patients with MDD.
Massachusetts General Hospital is the original and largest clinical
education and research facility of Harvard Medical School/Harvard
University and houses the world's largest hospital-based research
program.
This press release features multimedia. View
the full release here:
https://www.businesswire.com/news/home/20240812438959/en/
Massachusetts General Hospital (Photo:
Business Wire)
Alzamend and Mass General have contracted with Dr. Ovidiu
Andronesi MD, PhD, to be the principal investigator on the study.
Dr. Andronesi is an Associate Professor of Radiology at Harvard
University and the Director of Multinuclear Metabolic Imaging,
Martinos Center for Biomedical Imaging, Department of Radiology,
Massachusetts General Hospital.
Lithium was the first mood stabilizer approved by the U.S. Food
and Drug Administration (“FDA”) and is still a first-line
treatment option (considered the “gold standard”) for BD. Moreover,
lithium has been marketed for more than 35 years and human
toxicology regarding its use has been well characterized,
potentially mitigating the regulatory burden for safety data.
Although lithium does not have an FDA approved indication for
augmentation of an antidepressant in MDD, it has been prescribed
off-label for this purpose for decades. While a wide variety of
medications have been used historically in this capacity, lithium
is one of the few agents that has demonstrated efficacy in multiple
randomized controlled trials. Although the ideal role for lithium
augmentation has yet to be established, there is evidence to
support the clinical practice of adding lithium to conventional
antidepressants in pursuit of MDD remission.
The objective of this novel trial is to assess the comparative
increase in lithium levels within the brain and its structures as
opposed to a commonly marketed lithium salt among MDD patients. By
examining the lithium content in the brains and brain structures of
patients during treatments, Alzamend aims to predict the minimum
dose necessary to achieve the equivalent effectiveness and safety
of AL001 in contrast to established lithium salts. Alzamend is
optimistic that this study will assist in meeting the regulatory
safety standards through the Section 505(b)(2) pathway for approval
by the U.S. Food and Drug Administration, which is specifically
designed for new formulations/ delivery systems of an approved
drug.
Alzamend previously completed a Phase IIA multiple ascending
dose clinical trial, in which it successfully identified a maximum
tolerated dose (“MTD”) for development of AL001, as assessed
by an independent safety review committee. This dose, providing
lithium at a lithium carbonate equivalent dose of 240 mg 3-times
daily, is designed to be unlikely to require lithium therapeutic
drug monitoring (“TDM”). Current FDA-approved lithium salts
(carbonate and citrate) are limited by a narrow therapeutic window
that requires regular TDM of plasma lithium levels and blood
chemistry by a clinician to mitigate adverse events. Since
conventional lithium salts are eliminated relatively quickly,
multiple administrations throughout the day are required to safely
reach therapeutic plasma concentrations. Existing lithium drugs
suffer from chronic toxicity, poor physicochemical properties, and
poor brain bioavailability. However, because lithium is so
effective, it is still used clinically despite its narrow
therapeutic index.
“We are elated to partner with Massachusetts General Hospital
and Dr. Andronesi in this pivotal study for our lead therapeutic
candidate AL001,” said Stephan Jackman, Chief Executive Officer of
Alzamend. “If we can develop a next-generation lithium product
(AL001) with an improved safety profile and enhanced
biodistribution in the brain that would not routinely require TDM,
it would constitute a major improvement over current lithium-based
treatments and positively impact the 21+ million Americans
afflicted with MDD. We look forward to providing more details
regarding the study’s timeline and market opportunity in the near
future.”
About AL001
AL001 is a novel lithium-delivery system that has the potential
to deliver benefits of marketed lithium salts while mitigating or
avoiding currently experienced toxicities associated with lithium.
Results from Alzamend’s completed Phase IIA multiple-ascending dose
study of AL001 in Alzheimer’s patients and healthy subjects
identified an MTD, as assessed by an independent safety review
committee. This MTD is designed to be unlikely to require TDM while
providing lithium at a relatively modest but effective dose. AL001
is designed to favorably distribute lithium in the brain resulting
in lower exposure of other body organs and an improved safety
profile compared to currently marketed lithium salts. This can
serve to mitigate or obviate the disadvantageously low ceiling for
toxicity of marketed lithium salts that has limited their
usefulness to patients and prescribers.
About Alzamend Neuro
Alzamend Neuro is a clinical-stage biopharmaceutical company
focused on developing novel products for the treatment of
Alzheimer’s, BD, MDD and PTSD. Our mission is to rapidly develop
and market safe and effective treatments. Our current pipeline
consists of two novel therapeutic drug candidates, AL001 - a
patented ionic cocrystal technology delivering lithium via a
therapeutic combination of lithium, salicylate and L-proline, and
ALZN002 - a patented method using a mutant-peptide sensitized cell
as a cell-based therapeutic vaccine that seeks to restore the
ability of a patient’s immunological system to combat Alzheimer’s
by removing beta-amyloid from the brain. The latter is a
second-generation active-immunity approach designed to mitigate the
disadvantages of approved passive immunity marketed antibody
products, particularly by reducing the required frequency and costs
of dosing associated with antibody products. Both of our product
candidates are licensed from the University of South Florida
Research Foundation, Inc. pursuant to royalty-bearing exclusive
worldwide licenses.
Forward-Looking Statements
This press release contains “forward-looking statements” within
the meaning of Section 27A of the Securities Act of 1933, as
amended, and Section 21E of the Securities Exchange Act of 1934, as
amended. These forward-looking statements generally include
statements that are predictive in nature and depend upon or refer
to future events or conditions, and include words such as
“believes,” “plans,” “anticipates,” “projects,” “estimates,”
“expects,” “intends,” “strategy,” “future,” “opportunity,” “may,”
“will,” “should,” “could,” “potential,” or similar expressions.
Statements that are not historical facts are forward-looking
statements. Forward-looking statements are based on current beliefs
and assumptions that are subject to risks and uncertainties.
Forward-looking statements speak only as of the date they are made,
and Alzamend undertakes no obligation to update any of them
publicly in light of new information or future events. Actual
results could differ materially from those contained in any
forward-looking statement as a result of various factors. More
information, including potential risk factors, that could affect
Alzamend’s business and financial results are included in
Alzamend’s filings with the U.S. Securities and Exchange
Commission. All filings are available at www.sec.gov and on
Alzamend’s website at www.Alzamend.com.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240812438959/en/
Email: Info@Alzamend.com or call: 1-844-722-6333
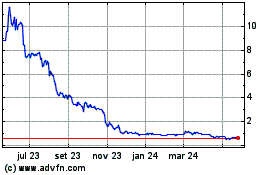
Alzamend Neuro (NASDAQ:ALZN)
Gráfico Histórico do Ativo
De Out 2024 até Nov 2024
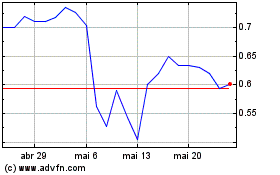
Alzamend Neuro (NASDAQ:ALZN)
Gráfico Histórico do Ativo
De Nov 2023 até Nov 2024