Pfizer Says FDA Approves Xeljanz XR Extended-Release Tablets to Treat Ulcerative Colitis
12 Dezembro 2019 - 8:43PM
Dow Jones News
By Stephen Nakrosis
Pfizer Inc. (PFE) said Thursday the U.S. Food and Drug
Administration approved Xeljanz extended-release tablets for adult
patients with moderately to severely active ulcerative colitis.
Pfizer said its extended-release 11 mg and 22 mg tablets of
Xeljanz, or tofacitinib, was indicated "for the once-daily
treatment of adult patients with moderately to severely active
ulcerative colitis, after an inadequate response or intolerance to
TNF blockers."
Xeljanz is also indicated for the treatment of Rheumatoid
Arthritis and Psoriatic Arthritis, the company said.
--Write to Stephen Nakrosis at stephen.nakrosis@wsj.com
(END) Dow Jones Newswires
December 12, 2019 18:28 ET (23:28 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
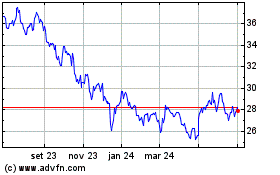
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Abr 2024 até Mai 2024
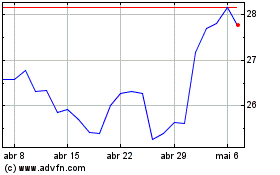
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Mai 2023 até Mai 2024