Abbott Labs Gets U.S. Clearance For Heart Mapping Platform
12 Janeiro 2022 - 11:41AM
Dow Jones News
By Will Feuer
Abbott Laboratories said Wednesday that it has received
clearance from the U.S. Food and Drug Administration for its new
heart mapping platform.
Abbott's EnSite X EP System with EnSite Omnipolar Technology
creates detailed three-dimensional maps of the heart to help
physicians better treat abnormal heart rhythms, or cardiac
arrhythmias, the company said.
"These models provide a way to precisely identify areas that are
causing problems, so physicians can better treat those abnormal
heart rhythms, and preserve healthy tissue," said Mike Pederson,
senior vice president of electrophysiology at Abbott.
Write to Will Feuer at Will.Feuer@wsj.com
(END) Dow Jones Newswires
January 12, 2022 09:26 ET (14:26 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
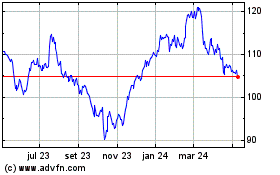
Abbott Laboratories (NYSE:ABT)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
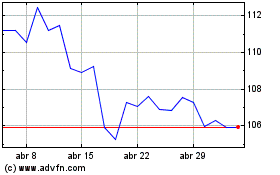
Abbott Laboratories (NYSE:ABT)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024