Erasca's Eras-801 for Treatment of Brain Tumors Gets FDA Orphan Drug Designation
22 Junho 2023 - 5:56PM
Dow Jones News
By Sabela Ojea
Erasca said Thursday that its Eras-801 medication aimed at
treating brain tumors was granted Orphan Drug Designation by the
U.S. Food and Drug Administration.
Shares rise 3.4% to $2.75 in after-hours trading.
The clinical-stage oncology company said Eras-801 has been
designated to treat aggressive brain tumors such as
Glioblastoma.
While orphan-drug status doesn't guarantee FDA approval as a
treatment for a rare disease, it is a prerequisite for getting
incentives such as tax breaks and marketing exclusivity if the
agency approves the drug for that purpose.
Write to Sabela Ojea at sabela.ojea@wsj.com; @sabelaojeaguix
(END) Dow Jones Newswires
June 22, 2023 16:41 ET (20:41 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
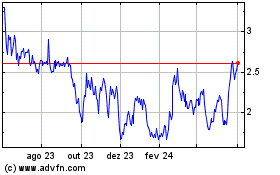
Erasca (NASDAQ:ERAS)
Gráfico Histórico do Ativo
De Abr 2024 até Mai 2024
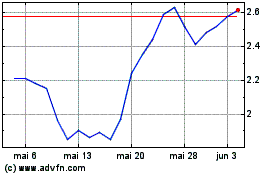
Erasca (NASDAQ:ERAS)
Gráfico Histórico do Ativo
De Mai 2023 até Mai 2024