GSK's Nucala Accepted for Review in Adult Use in Japan
01 Setembro 2023 - 3:47AM
Dow Jones News
By Anthony O. Goriainoff
GSK said Japan has accepted for review a supplementary new drug
application for its Nucala (mepolizumab) product for the treatment
of adults with chronic rhinosinusitis with nasal polyps.
The U.K. pharmaceutical giant said Friday that if approved,
mepolizumab would be the first anti-interleukin-5--a
pro-inflammatory protein--for adult patients in Japan.
GSK said this would be Nucala's third indication in Japan for an
interleukin-5 mediated condition.
The company said mepolizumab--an antibody targeting
interleukin-5--is already approved in Japan as a treatment for
bronchial asthma in children above the age of six, as well as
adults with refractory asthma whose symptoms are inadequately
controlled with standard treatments.
Write to Anthony O. Goriainoff at
anthony.orunagoriainoff@dowjones.com
(END) Dow Jones Newswires
September 01, 2023 02:32 ET (06:32 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
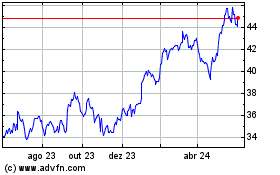
GSK (NYSE:GSK)
Gráfico Histórico do Ativo
De Abr 2024 até Mai 2024
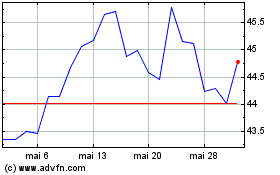
GSK (NYSE:GSK)
Gráfico Histórico do Ativo
De Mai 2023 até Mai 2024