Canada Approves Use of Moderna's Covid-19 Booster
12 Setembro 2023 - 1:05PM
Dow Jones News
By Robb M. Stewart
Canada's health regulator has authorized the use of Moderna's
latest Covid-19 booster targeting the Omicron XBB.1.5 subvariant
for anyone six months of age and older.
Health Canada said Tuesday it also is reviewing a submission
from Pfizer and partner BioNTech seeking approval for their own
Covid-19 vaccine for the subvariant for the same age group, and has
received a submission from Novavax for its vaccine targeting the
subvariant for people 12 and older.
The agency said that it determined Moderna's latest vaccine
meets safety, efficacy and quality requirements.
According to the product label for Moderna's vaccine, known as
Spikevax, anyone five years and older should receive one dose,
regardless of their Covid-19 vaccination history, while those
between six months and four years old should receive two doses if
they haven't previously had a Covid-19 vaccine or a single dose if
they have had one or more prior doses of a Covid-19 vaccine.
Health Canada's approval comes after the U.S. Food and Drug
Administration cleared the updated boosters from Pfizer and Moderna
for people six months and older on Monday. Advisers to the Centers
for Disease Control and Prevention are expected to endorse the
latest Covid-19 boosters on Tuesday afternoon.
Write to Robb M. Stewart at robb.stewart@wsj.com
(END) Dow Jones Newswires
September 12, 2023 11:50 ET (15:50 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
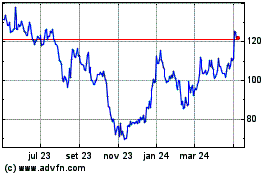
Moderna (NASDAQ:MRNA)
Gráfico Histórico do Ativo
De Jun 2024 até Jul 2024
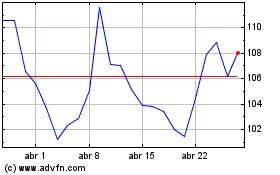
Moderna (NASDAQ:MRNA)
Gráfico Histórico do Ativo
De Jul 2023 até Jul 2024