Philips Spectral CT 7500 RT, world’s first spectral 4DCT respiratory-gated imaging receives FDA 510(k) clearance
12 Novembro 2024 - 11:00AM

November 12, 2024
Designed specifically for use in radiation oncology, new
Spectral CT 7500 RT enables personalized radiation therapy planning
to deliver better care for more cancer patients
Amsterdam, the Netherlands – Royal Philips
(NYSE: PHG, AEX: PHIA), a global leader in health technology, today
announced a major advance in radiation oncology with 510(k)
clearance from the US Food and Drug Administration (FDA) for its
new detector-based spectral computed tomography (CT) radiotherapy
solution. Philips Spectral CT 7500 RT marks the next step in
personalized cancer care by integrating the unique tumor
visualization and tissue characterization capabilities of spectral
CT into cancer treatment and planning. Radiation oncologists can
now precisely target radiation therapy to the specific
physiological characteristics of a patient's tumor, minimizing
damage to healthy surrounding tissue and reducing potential
unwanted side effects.
Spectral CT 7500 RT revolutionizes radiation oncology imaging by
combining true conventional and spectral CT capabilities in a
single scan, seamlessly integrating into existing clinical
workflows. As the first radiation therapy CT scanner to offer
respiratory-gated spectral imaging, radiation oncologists have all
the benefits of 4D conventional CT, and can also now apply the
improved visualization and quantification of spectral CT. This
latest innovation from Philips benefits radiotherapy departments by
reducing the costs of additional scans while enhancing accuracy and
enabling more effective treatment plans for a greater number of
cancer patients.
“Tumor delineation, beam attenuation, and respiratory motion are
critical factors in radiotherapy planning. The spectral information
provided by Spectral CT 7500 RT enhances tissue characterization,
enabling wider access to highly personalized and precisely targeted
treatment for more patients without adding extra steps to current
radiotherapy workflows,” said Dan Xu, Global Business Leader of CT
at Philips.
Spectral CT has been shown to reduce proton stopping-power ratio
(SPR) error by more than 50% compared to conventional CT, improving
the accuracy of radiation treatment and sparing healthy tissue [1].
Philips Spectral CT 7500 RT acquires both true conventional CT and
spectral CT information in a single scan. It can automatically
create the SPR map and direct electron density (ED) results with
less than 1% deviation [1] to enhance both the dose calculation and
accuracy of radiotherapy planning.
"The Spectral CT system provides us with several capabilities
that conventional CT does not have. It can provide electron density
and effective atomic number results, which we can convert to
the proton stopping-power ratio. And published data shows that
the stopping power ratio obtained in this way has fewer
uncertainties compared to regular calibration curves, thereby
reducing the uncertainty margins during treatment planning," said
Dr. Zhong Su, Physics Director, Department of Radiation Oncology at
the University of Arkansas for Medical Sciences (UAMS) Medical
Center (Arkansas, USA).
Philips CT has revolutionized medical imaging over the past four
decades, helping physicians make confident and precise diagnoses to
deliver better care for more people. The company is a recognized
leader in spectral CT with its ‘always-on’ detector-based
technology, providing spectral CT solutions across a wide range of
clinical areas including cardiology, oncology, neurology,
musculoskeletal, and pediatrics. For more information, visit
Philips at RSNA 2024.
[1] Longarino FK, Kowalewski A, Tessonnier T, et al. Potential
of a Second-Generation Dual-Layer Spectral CT for Dose Calculation
in Particle Therapy Treatment Planning. Front Oncol. 2022;12.
doi:10.3389/fonc.2022.853495
For further information, please contact:
Kathy O’ReillyPhilips Global External RelationsTel.: +1
978-221-8919E-mail: kathy.oreilly@philips.com
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health
technology company focused on improving people's health and
well-being through meaningful innovation. Philips’ patient- and
people-centric innovation leverages advanced technology and deep
clinical and consumer insights to deliver personal health solutions
for consumers and professional health solutions for healthcare
providers and their patients in the hospital and the home.
Headquartered in the Netherlands, the company is a leader in
diagnostic imaging, ultrasound, image-guided therapy, monitoring,
and enterprise informatics, as well as in personal health. Philips
generated 2023 sales of EUR 18.2 billion and employs approximately
69,300 employees with sales and services in more than 100
countries. News about Philips can be found at
www.philips.com/newscenter.
- Philips Spectral CT 7500 RT
- Spectral CT 7500 RT clinical
Koninklijke Philips NV (NYSE:PHG)
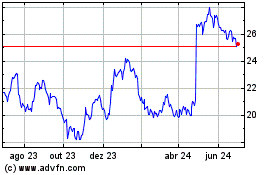
Gráfico Histórico do Ativo
De Out 2024 até Nov 2024
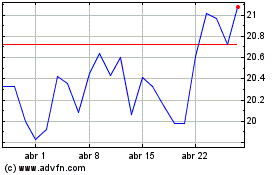
Koninklijke Philips NV (NYSE:PHG)
Gráfico Histórico do Ativo
De Nov 2023 até Nov 2024