- First and only respiratory syncytial virus (RSV) vaccine
indicated for adults younger than 50
- Approval based on data from pivotal Phase 3 trial in adults at
increased risk of lower respiratory tract disease caused by
RSV
Pfizer Inc. (NYSE: PFE) announced today that the U.S. Food and
Drug Administration (FDA) has approved ABRYSVO® (Respiratory
Syncytial Virus Vaccine), the company’s bivalent RSV prefusion F
(RSVpreF) vaccine, for the prevention of lower respiratory tract
disease (LRTD) caused by RSV in individuals 18 through 59 years of
age who are at increased risk for LRTD caused by RSV. ABRYSVO now
offers the broadest RSV vaccine indication for adults, which
previously included those 60 years and older. Additionally, it
remains the only RSV immunization approved for pregnant individuals
at 32 through 36 weeks of gestation to protect infants from birth
up to 6 months of age.
“RSV represents a significant threat to younger adults with
certain chronic conditions. After decades of vaccine research by
the scientific community and Pfizer, we now have the opportunity to
help alleviate the burden of RSV in this high-risk adult
population,” said Aamir Malik, Chief U.S. Commercial Officer and
Executive Vice President, Pfizer. “With this approval, we are proud
that ABRYSVO is now the only RSV vaccine indicated for adults aged
18 to 49 at increased risk for the disease, expanding on its
existing indications for older adults and pregnant women.”
The FDA’s decision is based on inferred efficacy1 from the
pivotal Phase 3 clinical trial (NCT05842967) MONeT (RSV
IMmunizatiON Study for AdulTs at Higher Risk
of Severe Illness), which investigated the safety, tolerability,
and immunogenicity of ABRYSVO in adults at risk of RSV-associated
disease due to certain chronic medical conditions. The company
intends to submit results from MONeT for publication in a
peer-reviewed scientific journal and for presentation at an
upcoming scientific conference.
Among U.S. adults 18 to 49 years of age, 9.5% have an underlying
chronic condition, such as obesity, diabetes, chronic obstructive
pulmonary disease (COPD), heart failure, chronic kidney disease,
and asthma2 that puts them at increased risk of developing, and
being hospitalized for, RSV-associated LRTD, and this rises to
24.3% among those 50 to 64 years of age.3,4
ABOUT RSV Respiratory syncytial virus (RSV) is a
contagious virus and a common cause of respiratory illness.5 The
virus can affect the lungs and breathing passages of an infected
individual, potentially causing severe illness or death.6,7 Chronic
cardiovascular disease, chronic lung disease, moderate or severe
immune compromise, diabetes with complications, and severe obesity
are among the conditions that increase an individual’s risk for
severe RSV.8 There are two major subgroups of RSV: RSV-A and RSV-B.
Both subgroups cause disease and can co-circulate or alternate
predominance from season to season.
ABOUT ABRYSVO Pfizer currently is the only company with
an RSV vaccine to help protect adults aged 60 and older, and adults
18 and older at increased risk of lower respiratory tract disease
caused by RSV (RSV-LRTD), as well as infants through maternal
immunization. ABRYSVO is an unadjuvanted, bivalent vaccine that was
designed to provide broad protection against RSV-LRTD, regardless
of the virus subgroup. In the prefusion state, the RSV fusion
protein (F) is a major target of neutralizing antibodies, serving
as the basis of Pfizer’s RSV vaccine. Variations in the F protein
sequence among RSV-A and RSV-B subgroups are clustered in a key
antigenic site, a target for potent neutralizing antibodies.
In May 2023, the FDA approved ABRYSVO for the prevention of LRTD
caused by RSV in individuals 60 years of age or older. In June
2024, the Advisory Committee on Immunization Practices (ACIP) voted
to update its recommendation of RSV vaccines for use in adults aged
≥75 years and adults age 60-74 years who are increased risk for
severe RSV disease. In August 2023, the FDA approved ABRYSVO for
the prevention of LRTD and severe LRTD caused by RSV in infants
from birth up to 6 months of age by active immunization of pregnant
individuals at 32 through 36 weeks gestational age. This was
followed in September 2023 with ACIP’s recommendation for maternal
immunization to help protect newborns from RSV seasonally where the
vaccine should be administered from September through January in
most of the continental United States.
Also in August 2023, Pfizer announced that the European
Commission granted marketing authorization for ABRYSVO for both
older adults and maternal immunization to help protect infants.
Additionally, ABRYSVO has received approvals for both indications
in multiple countries worldwide.
INDICATIONS FOR ABRYSVO
ABRYSVO® is a vaccine indicated in the US for:
- the prevention of lower respiratory tract disease (LRTD) caused
by respiratory syncytial virus (RSV) in people 60 years of age and
older
- the prevention of LRTD caused by RSV in people 18 through 59
years of age who are at increased risk for LRTD caused by RSV
- pregnant individuals at 32 through 36 weeks gestational age for
the prevention of LRTD and severe LRTD caused by RSV in infants
from birth through 6 months of age
IMPORTANT SAFETY INFORMATION FOR ABRYSVO
- ABRYSVO should not be given to anyone with a history of severe
allergic reaction (e.g., anaphylaxis) to any of its components
- For pregnant individuals: to avoid the potential risk of
preterm birth, ABRYSVO should be given during 32 through 36 weeks
gestational age
- Fainting can happen after getting injectable vaccines,
including ABRYSVO. Precautions should be taken to avoid falling and
injury during fainting
- Adults with weakened immune systems, including those receiving
medicines that suppress the immune system, may have a reduced
immune response to ABRYSVO
- Vaccination with ABRYSVO may not protect all people
- In adults 60 years of age and older, the most common side
effects (≥10%) were fatigue, headache, pain at the injection site,
and muscle pain
- In adults 18 through 59 years of age, the most common side
effects (≥10%) were pain at the injection site, muscle pain, joint
pain and nausea
- In pregnant individuals, the most common side effects (≥10%)
were pain at the injection site, headache, muscle pain, and
nausea
- In clinical trials where ABRYSVO was compared to placebo,
infants born to pregnant individuals experienced low birth weight
(5.1% ABRYSVO versus 4.4% placebo) and jaundice (7.2% ABRYSVO
versus 6.7% placebo)
View the full ABRYSVO Prescribing Information. If
it is not currently available via this link, it will be visible as
soon as possible as we work to finalize the document. Please check
back for the full information shortly.
About Pfizer: Breakthroughs That Change Patients’ Lives
At Pfizer, we apply science and our global resources to bring
therapies to people that extend and significantly improve their
lives. We strive to set the standard for quality, safety and value
in the discovery, development and manufacture of health care
products, including innovative medicines and vaccines. Every day,
Pfizer colleagues work across developed and emerging markets to
advance wellness, prevention, treatments and cures that challenge
the most feared diseases of our time. Consistent with our
responsibility as one of the world's premier innovative
biopharmaceutical companies, we collaborate with health care
providers, governments and local communities to support and expand
access to reliable, affordable health care around the world. For
175 years, we have worked to make a difference for all who rely on
us. We routinely post information that may be important to
investors on our website at www.Pfizer.com. In addition, to learn
more, please visit us on www.Pfizer.com and follow us on X at
@Pfizer and @Pfizer News, LinkedIn, YouTube and like us on Facebook
at Facebook.com/Pfizer.
DISCLOSURE NOTICE: The information contained in this
release is as of October 22, 2024. Pfizer assumes no obligation to
update forward-looking statements contained in this release as the
result of new information or future events or developments.
This release contains forward-looking information about ABRYSVO,
including its potential benefits and an approval in the U.S. for
the prevention of LRTD caused by RSV in individuals 18 through 59
years of age who are at increased risk for LRTD caused by RSV, that
involves substantial risks and uncertainties that could cause
actual results to differ materially from those expressed or implied
by such statements. Risks and uncertainties include, among other
things, uncertainties regarding the commercial success of ABRYSVO;
the uncertainties inherent in research and development, including
the ability to meet anticipated clinical endpoints, commencement
and/or completion dates for our clinical trials, regulatory
submission dates, regulatory approval dates and/or launch dates, as
well as the possibility of unfavorable new clinical data and
further analyses of existing clinical data; risks associated with
interim data; the risk that clinical trial data are subject to
differing interpretations and assessments by regulatory
authorities; whether regulatory authorities will be satisfied with
the design of and results from our clinical studies; whether and
when biologic license applications may be filed in particular
jurisdictions for ABRYSVO for any potential indications; whether
and when any applications that may be pending or filed for ABRYSVO
may be approved by regulatory authorities, which will depend on
myriad factors, including making a determination as to whether the
product's benefits outweigh its known risks and determination of
the product's efficacy and, if approved, whether ABRYSVO for any
such indications will be commercially successful; intellectual
property and other litigation; decisions by regulatory authorities
impacting labeling, manufacturing processes, safety and/or other
matters that could affect the availability or commercial potential
of ABRYSVO; uncertainties regarding the ability to obtain
recommendations from vaccine advisory or technical committees and
other public health authorities regarding ABRYSVO and uncertainties
regarding the commercial impact of any such recommendations;
uncertainties regarding the impact of COVID-19 on our business,
operations and financial results; and competitive developments.
A further description of risks and uncertainties can be found in
Pfizer’s Annual Report on Form 10-K for the fiscal year ended
December 31, 2023, and in its subsequent reports on Form 10-Q,
including in the sections thereof captioned “Risk Factors” and
“Forward-Looking Information and Factors That May Affect Future
Results”, as well as in its subsequent reports on Form 8-K, all of
which are filed with the U.S. Securities and Exchange Commission
and available at www.sec.gov and www.pfizer.com.
_______________________________ REFERENCES 1 Fink D. Immunobridging to
Evaluate Vaccines.
https://cdn.who.int/media/docs/default-source/blue-print/doran-fink_4_immunobridging_vrconsultation_6.12.2021.pdf.
Published June 2021, Accessed October 2024 2 U.S. Centers for
Disease Control and Prevention. “Morbidity and Mortality Weekly
Report.” August 15, 2024.
https://www.cdc.gov/mmwr/volumes/73/wr/mm7332e1.htm 3 Weycker, D.,
Averin, A., Houde, L. et al. Rates of Lower Respiratory Tract
Illness in US Adults by Age and Comorbidity Profile. Infect Dis
Ther 2024;13: 207–220. doi: 10.1007/s40121-023-00904-z. Epub 2024
Jan 18. PMID: 38236516; PMCID: PMC10828164. 4 U.S. Centers for
Disease Control and Prevention. “Epidemiology of Respiratory
Syncytial Virus Hospitalizations in Adults — RSV-NET.”
https://www.cdc.gov/acip/downloads/slides-2023-10-25-26/03-Patton-Adult-RSV-508.pdf.
Presented October 25, 2023. Accessed October 2024. 5 World Health
Organization. Respiratory Syncytial Virus (RSV) disease.
https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/norms-and-standards/vaccine-standardization/respiratory-syncytial-virus-disease.
Accessed October 2024. 6 U.S. Centers for Disease Control and
Prevention. How RSV Spreads.
https://www.cdc.gov/rsv/causes/index.html. Updated August 30, 2024.
Accessed October 2024. 7 U.S. Centers for Disease Control and
Prevention. RSV in Older Adults.
https://www.cdc.gov/rsv/older-adults/index.html. Updated August 30,
2024. Accessed October 2024. 8 U.S. Centers for Disease Control and
Prevention. Clinical Overview of RSV.
https://www.cdc.gov/rsv/hcp/clinical-overview/index.html. Updated
August 30, 2024. Accessed October 2024.
Category: Vaccines
View source
version on businesswire.com: https://www.businesswire.com/news/home/20241018199265/en/
Media Contact: PfizerMediaRelations@Pfizer.com +1 (212)
733-1226
Investor Contact: IR@Pfizer.com +1 (212) 733-4848
Pfizer (NYSE:PFE)
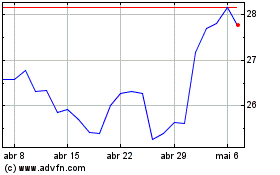
Gráfico Histórico do Ativo
De Nov 2024 até Dez 2024
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
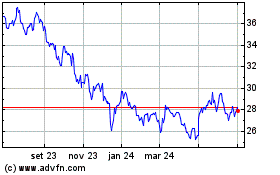
De Dez 2023 até Dez 2024