UPDATE: FDA: Possible Association Between Breast Implants, Rare Cancer
26 Janeiro 2011 - 7:02PM
Dow Jones News
The Food and Drug Administration is investigating a possible
link between breast implants and a rare type of cancer, reopening a
debate about the safety of implants.
Specifically the FDA is looking at reports of a type of lymphoma
called anaplastic large cell lymphoma that's mostly been found
adjacent to the breast implant. ALCL is a type of non-Hodgkin's
lymphoma and is not breast cancer.
The FDA said it's aware of just 60 cases since 1997 of ALCL in
women with both silicone and saline implants, a small number
compared to the estimated 5 million to 10 million women worldwide
who have implants.
ALCL appears in different parts of the body including the lymph
nodes and skin, according to the National Cancer Institute. Each
year ALCL is diagnosed in about 1 out of 500,000 women in the U.S.
ALCL in the breast is even more rare with 3 out of 100 million
women per year diagnosed in the U.S., FDA said.
"Data reviewed by the FDA suggest that patients with breast
implants may have a very small but significant risk of ALCL in the
scar capsule adjacent to the implant," the agency said in a
statement. But because the risk of ALCL appears to be "very small,"
FDA said it believes there's a "reasonable assurance" that implants
are safe and effective.
In the U.S., breast implants are sold by Allergan Inc. (AGN) and
Mentor Worldwide LLC, a unit of Johnson & Johnson (JNJ).
An Allergan spokeswoman said, "Patient safety is Allergan's
absolute first priority and we continue all efforts to collect and
analyze further information about the very rare occurrence of ALCL
in patients with breast implants."
A spokesman for Mentor said, the company "fully supports FDA's
efforts to gather additional information and study ALCL in patients
with breast implants.
William Maisel, FDA's chief scientist and a deputy director in
the agency's device division, urged doctors to notify the FDA about
any confirmed cases of ALCL in patients with implants. He said the
agency is working to establish an ALCL patient registry to better
establish if there's a link between implants and that type of
cancer.
The FDA said it started a review of ALCL and implants after
receiving case reports. The FDA said it identified 34 cases of ALCL
from 1997 through May 2010 from the medical literature. The agency
then contacted other regulatory agencies, scientists and the
companies to find a total of 60 cases. However, the FDA stressed
that some case reports might be duplicates.
The agency said it would work with Allergan and Mentor to update
labeling materials for patients and health care providers to
discuss the possible risk of ALCL.
Of the 34 reports seen in the medical literature, 24 were in
women with silicone implants and seven were reported in women with
saline implants. In three cases the type of implant wasn't
identified.
One theory the FDA is looking at is whether the silicone itself
might play a role in ALCL. Maisel said both types of implants are
"surrounded" by silicone.
The FDA sharply curbed the use of silicone-gel implants in 1992
after concerns were raised about whether leaking silicone gel
caused serious health problems, requiring women who wanted to
increase their breast size for cosmetic purposes alone to use
saline implants. In 2006 silicone implants were allowed back on the
market for cosmetic use to women age 22 and older.
Dow Corning Inc., a former silicone-gel implant maker, filed for
bankruptcy protection in 1995 after thousands of lawsuits over its
implants. The company later emerged from bankruptcy protection
started paying out $2.35 billion in claims to thousands of women
who sued the company over allegations they were injured by silicone
implants. Dow Corning is equally owned by Dow Chemical Co. (DOW)
and Corning Inc. (GLW).
-By Jennifer Corbett Dooren, Dow Jones Newswires; 202-862-9294;
jennifer.corbett@dowjones.com
Corning (NYSE:GLW)
Gráfico Histórico do Ativo
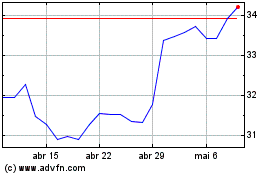
De Jun 2024 até Jul 2024
Corning (NYSE:GLW)
Gráfico Histórico do Ativo
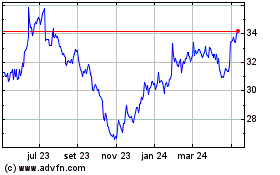
De Jul 2023 até Jul 2024