Janssen Seeks FDA Approval of New Darzalex Subcutaneous Formulation
12 Julho 2019 - 1:38PM
Dow Jones News
By Stephen Nakrosis
The Janssen Pharmaceutical Companies of Johnson & Johnson
(JNJ) said Friday it submitted an application with the U.S. Food
and Drug Administration seeking approval of its new subcutaneous
formulation of Darzalex, or daratumumab, for certain patients with
multiple myeloma.
Janssen said the Biologics License Application was supported by
data from its Phase 3 Columba study, which included 522 patients,
and data from the Phase 2 Pleiades study, which included 240
patients.
"The Darzalex subcutaneous formulation showed non-inferiority to
the existing IV formulation, both as a monotherapy and in
combination with common background therapies, while administered
with a considerably shorter infusion time. We look forward to
working closely with the FDA in their review of the data supporting
this regulatory application," said Craig Tendler, vice president,
clinical development and global medical affairs, oncology, Janssen
Research & Development.
Write to Stephen Nakrosis at stephen.nakrosis@wsj.com
(END) Dow Jones Newswires
July 12, 2019 12:23 ET (16:23 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
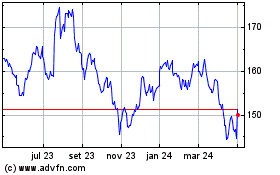
Johnson and Johnson (NYSE:JNJ)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
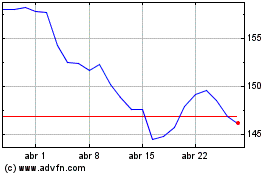
Johnson and Johnson (NYSE:JNJ)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024