Johnson & Johnson's Janssen Says Psoriatic Arthritis Trial Meets Objective
03 Dezembro 2021 - 11:04AM
Dow Jones News
By Michael Dabaie
Johnson & Johnson's Janssen Pharmaceutical Cos. said a study
of Tremfya met its primary objective in psoriatic arthritis.
The company said the latest Phase 3 data for Tremfya
demonstrated significant and durable improvement in signs and
symptoms of active psoriatic arthritis while maintaining its safety
profile in patients with inadequate response to tumor necrosis
factor inhibition.
Results showed significantly higher proportions of patients
treated with Tremfya had improvement in joint signs and symptoms
and complete skin clearance versus placebo at week 24. Improvements
in signs and symptoms of PsA were maintained or numerically
increased through one year among Tremfya-randomized patients, the
company said.
Tremfya is approved in the U.S. for moderate to severe plaque
psoriasis and active psoriatic arthritis.
Write to Michael Dabaie at michael.dabaie@wsj.com
(END) Dow Jones Newswires
December 03, 2021 08:49 ET (13:49 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
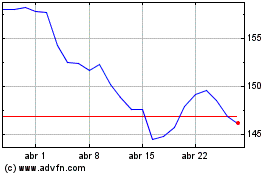
Johnson and Johnson (NYSE:JNJ)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
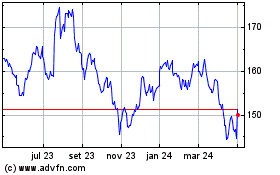
Johnson and Johnson (NYSE:JNJ)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024