Moderna: Health Canada Authorizes Omicron-Targeting Vaccine in 6-to-17-Year-Olds
17 Fevereiro 2023 - 1:46PM
Dow Jones News
By Denny Jacob
Moderna Inc. on Friday said Health Canada authorized the use of
its Omicron-targeting bivalent Covid-19 booster vaccine in children
and adolescents 6-to-17-years old, bringing the offering to a
greater portion of the population.
The pharmaceutical company said the pediatric and adolescent
authorization is based on clinical trial booster data for its
original vaccine, Spikevax, which was administered to over a
thousand participants in each cohort.
Moderna's Omicron-targeting bivalent vaccines are currently
authorized for use in individuals 18 years and older in Canada.
"The authorization of a booster dose of mRNA-1273.214 in
children and adolescents is a critical step to broaden protection
against the Omicron family of variants, and the emergence of new
variants of concern in Canada," said Chief Executive Stéphane
Bancel.
Write to Denny Jacob at denny.jacob@wsj.com
(END) Dow Jones Newswires
February 17, 2023 11:31 ET (16:31 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
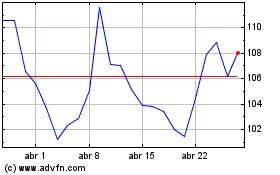
Moderna (NASDAQ:MRNA)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
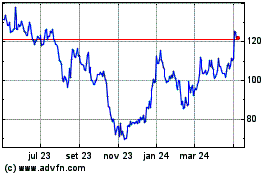
Moderna (NASDAQ:MRNA)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024