Replimune to Present at the 39th Annual Meeting of the Society for Immunotherapy of Cancer (SITC)
30 Outubro 2024 - 10:15AM

Replimune Group, Inc. (NASDAQ: REPL), a clinical stage
biotechnology company pioneering the development of novel oncolytic
immunotherapies, today announced that a late-breaking abstract
featuring the IGNYTE clinical trial primary analysis has been
selected for oral presentation at the 39th Annual Meeting of the
Society for Immunotherapy of Cancer (SITC 2024) being held November
6-10, 2024, in Houston, Texas. Data from the ARTACUS clinical trial
of RP1 monotherapy in solid organ transplant patients with advanced
cutaneous malignancies will also be shared in an encore poster
presentation.
Presentation Details
Title: Primary analysis of the
registration-intended cohort of patients with anti-PD1 failed
melanoma from the IGNYTE trial of RP1 plus nivolumab, including
clinical subgroup and initial biomarker dataAbstract
Number: 1504Presentation Session Title:
Session 204 – Clinical Late-Breaking Abstract SessionDate
and Time: Saturday, November 9, 2024, 11:45 am – 12:45 pm
CSTPresenter: Michael K. Wong, MD, PhD, University
of Texas MD Anderson Cancer Center
Title: Safety and efficacy results from an
open-label phase 1b/2 study of RP1 oncolytic immunotherapy in solid
organ transplant recipients with advanced cutaneous malignancies
(ARTACUS)Abstract Number: 693Date and
Time: Friday, November 8, 2024Presenter:
Diwakar Davar, MD, UPMC Hillman Cancer Center
About Replimune Replimune
Group, Inc., headquartered in Woburn, MA, was founded in 2015
with the mission to transform cancer treatment by pioneering the
development of novel oncolytic immunotherapies. Replimune’s
proprietary RPx platform is based on a potent HSV-1 backbone
intended to maximize immunogenic cell death and the induction of a
systemic anti-tumor immune response. The RPx platform is designed
to have a unique dual local and systemic activity consisting of
direct selective virus-mediated killing of the tumor resulting in
the release of tumor derived antigens and altering of the tumor
microenvironment to ignite a strong and durable systemic response.
The RPx product candidates are expected to be synergistic with most
established and experimental cancer treatment modalities, leading
to the versatility to be developed alone or combined with a variety
of other treatment options. For more information, please
visit www.replimune.com.
Forward Looking StatementsThis press release
contains forward looking statements within the meaning of Section
27A of the Securities Act of 1933, as amended, and Section 21E of
the Securities Exchange Act of 1934, as amended, including
statements regarding the design and advancement of our clinical
trials, the timing and sufficiency of our clinical trial outcomes
to support potential approval of any of our product candidates, our
goals to develop and commercialize our product candidates, patient
enrollments in our existing and planned clinical trials and the
timing thereof, and other statements identified by words such as
“could,” “expects,” “intends,” “may,” “plans,” “potential,”
“should,” “will,” “would,” or similar expressions and the negatives
of those terms. Forward-looking statements are not promises or
guarantees of future performance, and are subject to a variety of
risks and uncertainties, many of which are beyond our control, and
which could cause actual results to differ materially from those
contemplated in such forward-looking statements. These factors
include risks related to our limited operating history, our ability
to generate positive clinical trial results for our product
candidates, the costs and timing of operating our in-house
manufacturing facility, the timing and scope of regulatory
approvals, the availability of combination therapies needed to
conduct our clinical trials, changes in laws and regulations to
which we are subject, competitive pressures, our ability to
identify additional product candidates, political and global macro
factors including the impact of the coronavirus as a global
pandemic and related public health issues and the Russian-Ukrainian
and Israel-Hamas political and military conflicts, and other risks
as may be detailed from time to time in our Annual Reports on Form
10-K and Quarterly Reports on Form 10-Q and other reports we file
with the Securities and Exchange Commission. Our actual
results could differ materially from the results described in or
implied by such forward-looking statements. Forward-looking
statements speak only as of the date hereof, and, except as
required by law, we undertake no obligation to update or revise
these forward-looking statements.
Investor InquiriesChris BrinzeyICR
Westwicke339.970.2843chris.brinzey@westwicke.com
Media InquiriesArleen
GoldenbergReplimune917.548.1582media@replimune.com
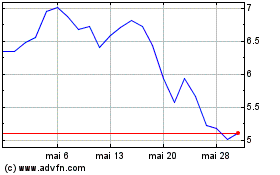
Replimune (NASDAQ:REPL)
Gráfico Histórico do Ativo
De Out 2024 até Nov 2024
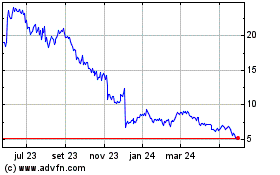
Replimune (NASDAQ:REPL)
Gráfico Histórico do Ativo
De Nov 2023 até Nov 2024