Amarin Receives National Reimbursement for VAZKEPA® in Italy
16 Dezembro 2024 - 9:00AM

Amarin Corporation plc (NASDAQ:AMRN), today announced that Italy’s
National Health Service (NHS) approved VAZKEPA® for national
reimbursement to reduce cardiovascular risk in eligible high-risk
patients, as published in the Official Journal of the Italian
Republic (i.e. Gazzetta Ufficiale della Repubblica Italiana)1.
As in most European countries, cardiovascular
disease is the leading cause of death and hospitalization in Italy,
with over 217,000 deaths2 per year and over 107,000
hospitalizations annually due to myocardial infarction alone3. One
in five cardiovascular patients experience another cardiovascular
event within 12 months of their first event4, highlighting the
urgent need for new treatment options to reduce cardiovascular
risk.
Commenting on the reimbursement, Dr Aldo Pietro
Maggioni, Director of the Study Center of the Italian Association
of Hospital Cardiologists (centro studi ANMCO) stated: “Patients
with established cardiovascular disease and elevated triglycerides,
despite a well-managed LDL-C level, still have a higher likelihood
of experiencing subsequent CV events, as there are currently no
effective therapies for this patient phenotype. The Italian
regulatory authority addressed this clinical need by authorizing
the reimbursement of icosapent ethyl which, in the REDUCE-IT study,
has shown to reduce cardiovascular death as well as recurrent
myocardial infarction, stroke and coronary revascularization. The
reimbursement approval of icosapent ethyl offers clinicians an
additional effective treatment option for high cardiovascular risk
patients.”
Italian Approval Underscores Impact
& Progress of New Strategy
As highlighted in Amarin’s Investor Day in
November, in 2023 the Company implemented a more focused strategic
approach to advance reimbursement, access and commercialization in
Europe. This improved strategy has delivered meaningful results.
Over the last 18 months, and factoring in the Italy approval, the
Company has now secured national reimbursement in 3 of the EU5
markets and nine European markets overall. This represents more
than 50% of the current total eCVD eligible patient population
across Western Europe.
Additionally, as a reminder, the intellectual
property for VAZKEPA in Europe has recently been extended to 2039.
Unlike the US, there is no “skinny label” risk that permits
competitive entry prior to the 2039 expiration of the Company’s
patents for the cardiovascular risk indication for VAZKEPA.
Commenting on the Italian approval, Aaron Berg,
President & CEO of Amarin, said, “Today is an important day,
not only for Amarin, but for patients in Italy, the third largest
economy in Europe. We appreciate that the Italian authorities have
recognized the strength of the clinical data supporting VAZKEPA and
affirming the potential impact it can have for the many eCVD
patients across all of Italy.”
Commenting on the Company’s progress in Europe
and globally, Berg said, “Today’s announcement is another important
step that builds upon the strong foundation to capitalize on the
tremendous untapped opportunity for VAZKEPA globally. With recently
granted patent protection extending the exclusivity in Europe to
2039, it is still early in the lifecycle with a long runway to
generate growth. Backed by strong science validated through the
endorsement of over 50 medical societies globally, and with 46
countries that have now approved VASCEPA/VAZKEPA for cardiovascular
risk reduction, we know there remains significant potential to
benefit millions of patients worldwide. Our focus remains clear: to
capitalize on the global value opportunity for VASCEPA/VAZKEPA by
getting it into the hands of as many patients as possible. That is
our commitment to patients, providers, employees and
shareholders.”
About Amarin
Amarin is an innovative pharmaceutical company
leading a new paradigm in cardiovascular disease management. We are
committed to increasing the scientific understanding of the
cardiovascular risk that persists beyond traditional therapies and
advancing the treatment of that risk for patients worldwide. Amarin
has offices in Bridgewater, New Jersey in the United States, Dublin
in Ireland, Zug in Switzerland, and other countries in Europe as
well as commercial partners and suppliers around the world.
Forward-Looking Statements
This press release contains forward-looking
statements which are made pursuant to the safe harbor provisions of
the Private Securities Litigation Reform Act of 1995, including
beliefs about the potential for VASCEPA (marketed as VAZKEPA in
Europe); beliefs about icosapent ethyl (IPE)’s role concerning
appropriate patients suffering from cardiovascular disease (CVD)
and potential population health impact, as well as general
beliefs about the safety and effectiveness of VASCEPA. These
forward-looking statements are not promises or guarantees and
involve substantial risks and uncertainties. A further list and
description of these risks, uncertainties and other risks
associated with an investment in Amarin can be found in Amarin's
filings with the U.S. Securities and Exchange Commission, including
Amarin’s annual report on Form 10-K for the full year ended 2023.
Existing and prospective investors are cautioned not to place undue
reliance on these forward-looking statements, which speak only as
of the date they are made. Amarin undertakes no obligation to
update or revise the information contained in its forward-looking
statements, whether as a result of new information, future events
or circumstances or otherwise. Amarin’s forward-looking statements
do not reflect the potential impact of significant transactions the
company may enter into, such as mergers, acquisitions,
dispositions, joint ventures or any material agreements that Amarin
may enter into, amend or terminate.
Availability of Other Information About
Amarin Amarin communicates with its investors and the
public using the company website (www.amarincorp.com) and the
investor relations website (investor.amarincorp.com), including but
not limited to investor presentations and FAQs, Securities and
Exchange Commission filings, press releases, public conference
calls and webcasts. The information that Amarin posts on these
channels and websites could be deemed to be material information.
As a result, Amarin encourages investors, the media and others
interested in Amarin to review the information that is posted on
these channels, including the investor relations website, on a
regular basis. This list of channels may be updated from time to
time on Amarin’s investor relations website and may include social
media channels. The contents of Amarin’s website or these channels,
or any other website that may be accessed from its website or these
channels, shall not be deemed incorporated by reference in any
filing under the Securities Act of 1933.
Amarin Contact
InformationInvestor & Media Inquiries:Mark
MarmurAmarin Corporation plcPR@amarincorp.com
1
https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2024-12-14&atto.codiceRedazionale=24A06678&elenco30giorni=false
2 Lstat 2021 -
https://www.istat.it/wp-content/uploads/2024/06/Report-cause-di-morte-Anno-2021.pdf
3 PNE 2022
4 Gargiulo G. et al. 2022
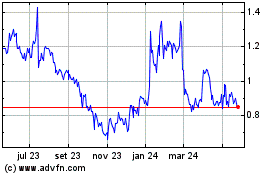
Amarin (NASDAQ:AMRN)
Gráfico Histórico do Ativo
De Nov 2024 até Dez 2024
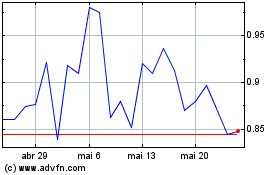
Amarin (NASDAQ:AMRN)
Gráfico Histórico do Ativo
De Dez 2023 até Dez 2024